Bcs Class
Posted on
Views
 Actions
Actions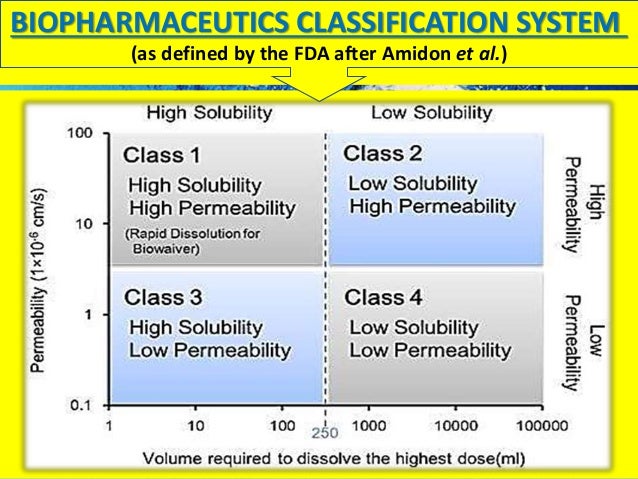
Whenever a new drug moiety is discovered, one of the 1st questions a pharmaceutical company asks is….whether or not the drug can be effectively administered by the oral route, for its intended use
Biopharmaceutical Classification System (BCS) is a scientific framework for classifying drug substances based on their aqueous solubility and intestinal permeability
Biopharmaceutical Classification System (BCS) is a scientific framework for classifying drug substances based on their aqueous solubility and intestinal permeability
BCS was originally used to grant biowaivers for scale-up and post-approval changes for drug products, but was later extended to the approval of new generic products.
FDA suggests as a potential internal standard
(Determination in Caco II cells only applicable to passively absorbed substances) A drug substance is considered “highly soluble” when the highest clinical dose strength is soluble in 250 mL or less of aqueous media over a pH range of 1–7.5 at 37 °C
when the dissolution rate is much greater than the gastric emptying, dissolution is not likely to be rate-limitingBCS Can be used to obtain a biowaiver It has been estimated that the application of BCS can result in annual savings of $35 million for
the pharmaceutical industry Reference: Particle size; Drug development services; Technical Brief 2011 Volume 9
22,320

From Embeds
Number of Embeds
Windows 10 get serial number. J Control Release. 2017 Feb 28;248:71-95. Doi: 10.1016/j.jconrel.2017.01.014. Epub 2017 Jan 11. BCS class IV drugs: Highly notorious candidates for.
Mp4 movie download in hindi. Step 3: Begin Downloading the HD MP4 Bollywood Movie Specify a destination folder to save the downloaded MP4 HD Hindi/Bollywood movie and click 'Download' button to start free downloading HD MP4 Bollywood/Hindi movies in Tamil/Telugu/English, etc. You can achieve old/latest Bollywood movies free download in HD, FLV, 3GP, WebM formats.
Bcs Class 2 Drugs List
 Actions
ActionsDownloads
Comments

Likes
Embeds 0
Biopharmaceutical Classification System (BCS) is a scientific framework for classifying drug substances based on their aqueous solubility and intestinal permeability
when the dissolution rate is much greater than the gastric emptying, dissolution is not likely to be rate-limiting
the pharmaceutical industry